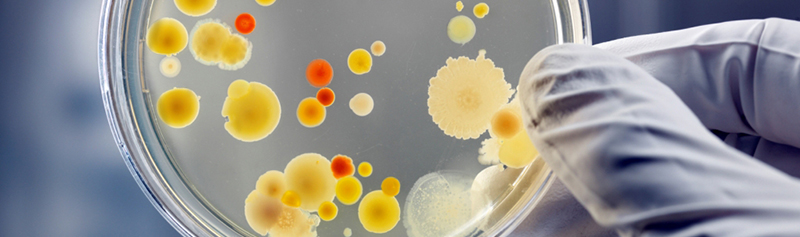
Isolates can be identified to genus or species level, depending on specific requirements, Grade of area under test and criticality in manufacturing process. Identification is important to assess the origin of contamination and to formulate corrective actions for contamination events. A minimum of Gram stain testing is recommended to establish possible source of contamination (e.g. Gram positive cocci are frequently Operator derived, whereas Gram negative rods may reveal an unknown water spillage/leak).
Simple biochemical testing, such as catalase and oxidase testing, and further stain manipulation, such as spore staining, can give further insight into the range of microorganisms isolated from the clean room and indicate specific requirements for their elimination (e.g. Gram positive sporing rods generally require treatment with Biocide). The undesirable organism S. aureus can be ruled out within minutes with a simple agglutination test and finally, most isolates can be easily identified to species level using API identification strips.
Full advice is offered at all stages of further testing so results are fully explained and only tests required by the Client are actually performed. In addition, we have a full Quality Management System which we can use to assist Clients with non-conformance investigations, reporting of Out of Specification (OOS) results and implementing Corrective and Preventative Actions (CAPAs).